
Physiology
Basic Cellular
Which of the following is the most potent stimulus of fibrinolysis:
Answer:
Fibrinolysis is a normal haemostatic response to vascular injury. Plasminogen, a proenzyme in blood and tissue fluid, is converted to plasmin by activators either from the vessel wall (intrinsic activation) or from the tissues (extrinsic activation). The most important route follows the release of tissue plasminogen activator (TPA) from endothelial cells.Haemostasis
Physiology / Basic Cellular / Blood and Blood Flow
Last Updated: 13th February 2026
Vasoconstriction
Immediate vasoconstriction of the injured vessel and reflex constriction of adjacent small arteries and arterioles is responsible for an initial slowing of blood flow to the area of injury. Where there is widespread damage, this vascular reaction prevents exsanguination. The reduced blood flow allows contact activation of platelets and coagulation factors.
Platelet Reactions and Primary Haemostatic Plug Formation
Following a break in the endothelial lining, there is an initial adherence of platelets (via GPIa and GPIb receptors) to exposed connective tissue, mediated by von Willebrand factor (VWF). Under conditions of high shear stress (e.g. arterioles), the exposed subendothelial matrix is initially coated with VWF. Collagen exposure and thrombin generated through activation of tissue factor produced at the site of injury cause the adherent platelets to release their granule contents and also activate platelet prostaglandin synthesis, leading to the formation of thromboxane A2 (TXA2). Released ADP causes platelets to swell and aggregate; TXA2 and serotonin (5-HT) also enhance the vasoconstriction.
Platelet rolling in the direction of blood flow over exposed VWF with activation of GPIIb/IIIa receptors results in firmer binding. Additional platelets from the circulating blood are drawn to the area of injury. This continuing platelet aggregation promotes the growth of the haemostatic plug which soon covers the exposed connective tissue. The unstable primary haemostatic plug produced by these platelet reactions in the first minute or so following injury is usually sufficient to provide temporary control of bleeding. The highly localised enhancement of platelet activation by ADP and TXA2 results in a platelet mass large enough to plug the area of endothelial injury.
Stabilisation of the Platelet Plug by Fibrin
Definitive haemostasis is achieved when fibrin, formed by blood coagulation, is added to the platelet mass and by platelet-induced clot retraction. Vascular injury results in the initiation and amplification of the coagulation cascade. Platelet aggregation and release reactions accelerate the coagulation cascade by providing abundant membrane phospholipid. The thrombin generated by the coagulation cascade converts soluble plasma fibrinogen into fibrin monomers, potentiates platelet aggregation and secretion and also activates factor XI and XIII and cofactors V and VIII. The fibrin monomers spontaneously polymerise to a fibrous mesh of fibrin, entrapping the platelets and other blood cells. The fibrin polymer is finally cross-linked by factor XIIIa to create a tough network of fibrin fibres, and a stable clot. Clot retraction occurs, mediated by GPIIb/IIIa receptors which link the cytoplasmic actin filaments to surface-bound fibrin polymers, making it tougher and assisting repair by drawing the edges of the wound together.

Haemostasis. (Image by OpenStax College [CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)])
Physiological Limitation of Blood Coagulation
Unchecked, blood coagulation would lead to thrombosis, of the protective mechanisms of coagulation inhibitors, blood flow and fibrinolysis were not in operation.
Coagulation Factor Inhibitors:
It is important that the effect of thrombin is limited to the site of the injury. The first inhibitor to act is tissue factor pathway inhibitor (TFPI), which is synthesised in endothelial cells and is present in plasma and platelets, and accumulates at the site of injury caused by local platelet activation. TFPI inhibits Xa and VIIa and tissue factor. There is also direct inactivation of thrombin and other protease factors by other circulating inhibitors, of which antithrombin is the most potent. Heparin potentiates its action markedly.
Protein C and Protein S:
Protein C and protein S are inhibitors of coagulation cofactors V and VIII. Thrombomodulin on endothelial cells binds thrombin and converts it so it no longer cleaves fibrinogen but instead activates protein C, which is able to destroy activated factors V and VIII, thus preventing further thrombin formation. The action of protein C is enhanced by protein S, which binds protein C to the platelet surface. Activated protein C also enhances fibrinolysis by destroying plasma inhibitors of tissue plasminogen activator (tPA).
Blood Flow:
At the periphery of a damaged area of tissue, blood flow rapidly achieves dilution and dispersal of activated factors before fibrin formation has occurred. Undamaged endothelium produces prostacyclin and nitric oxide which impede platelet adhesion and activation.
Fibrinolysis:
Fibrinolysis is a normal haemostatic response to vascular injury. Plasminogen, a proenzyme in blood and tissue fluid, is converted to plasmin by activators either from the vessel wall (intrinsic activation) or from the tissues (extrinsic activation). The most important route follows the release of tissue plasminogen activator (TPA) from endothelial cells.
TPA binds to fibrin which enhances its capacity to convert thrombus-bound plasminogen into plasmin. This fibrin dependence of TPA action strongly localises plasmin generation by TPA to the fibrin clot. Release of TPA occurs after such stimuli as trauma, exercise or emotional stress. Plasmin generation at the site of injury limits the extent of the evolving thrombus. The split products of fibrinolysis are also competitive inhibitors of thrombin and fibrin polymerisation.
Plasmin is capable of digesting fibrinogen, fibrin, factors V and VIII and many other proteins. Plasmin itself is inactivated by alpha2-antiplasmin. Cleavage of peptide bonds in fibrin and fibrinogen produces a variety of fibrin degradation products (FDPs). The D-dimer is a measurement of FDPs and is thus an indication of sequential thrombin and then plasmin activity. D-dimer may be raised in infection, malignancy and pregnancy, as well as venous thromboembolism. Levels are very high in patients with DIC.
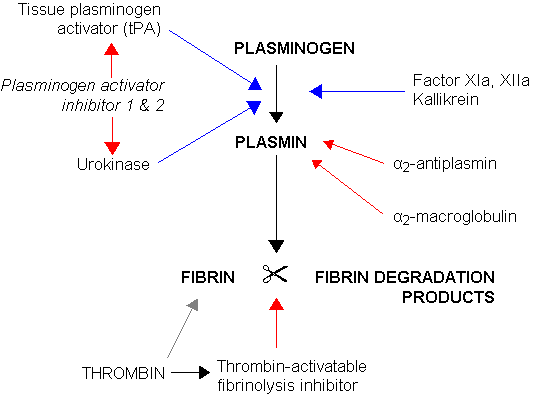
Fibrinolysis. (Image by Jfdwolff at en.wikipedia [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0/)])
Report A Problem
Is there something wrong with this question? Let us know and we’ll fix it as soon as possible.
Loading Form...
- Biochemistry
- Blood Gases
- Haematology
| Biochemistry | Normal Value |
|---|---|
| Sodium | 135 – 145 mmol/l |
| Potassium | 3.0 – 4.5 mmol/l |
| Urea | 2.5 – 7.5 mmol/l |
| Glucose | 3.5 – 5.0 mmol/l |
| Creatinine | 35 – 135 μmol/l |
| Alanine Aminotransferase (ALT) | 5 – 35 U/l |
| Gamma-glutamyl Transferase (GGT) | < 65 U/l |
| Alkaline Phosphatase (ALP) | 30 – 135 U/l |
| Aspartate Aminotransferase (AST) | < 40 U/l |
| Total Protein | 60 – 80 g/l |
| Albumin | 35 – 50 g/l |
| Globulin | 2.4 – 3.5 g/dl |
| Amylase | < 70 U/l |
| Total Bilirubin | 3 – 17 μmol/l |
| Calcium | 2.1 – 2.5 mmol/l |
| Chloride | 95 – 105 mmol/l |
| Phosphate | 0.8 – 1.4 mmol/l |
| Haematology | Normal Value |
|---|---|
| Haemoglobin | 11.5 – 16.6 g/dl |
| White Blood Cells | 4.0 – 11.0 x 109/l |
| Platelets | 150 – 450 x 109/l |
| MCV | 80 – 96 fl |
| MCHC | 32 – 36 g/dl |
| Neutrophils | 2.0 – 7.5 x 109/l |
| Lymphocytes | 1.5 – 4.0 x 109/l |
| Monocytes | 0.3 – 1.0 x 109/l |
| Eosinophils | 0.1 – 0.5 x 109/l |
| Basophils | < 0.2 x 109/l |
| Reticulocytes | < 2% |
| Haematocrit | 0.35 – 0.49 |
| Red Cell Distribution Width | 11 – 15% |
| Blood Gases | Normal Value |
|---|---|
| pH | 7.35 – 7.45 |
| pO2 | 11 – 14 kPa |
| pCO2 | 4.5 – 6.0 kPa |
| Base Excess | -2 – +2 mmol/l |
| Bicarbonate | 24 – 30 mmol/l |
| Lactate | < 2 mmol/l |

