
Physiology
Renal
Regarding acid-base regulation in the kidneys, which of the following statements is CORRECT:
Answer:
Bicarbonate is freely filtered at the glomerulus. Less than 0.1% of filtered bicarbonate is normally excreted in the urine (if plasma [HCO3-] increases, maximum tubular transport is exceeded and some HCO3- is excreted in urine). About 80% of bicarbonate is reabsorbed in the proximal tubule. For each H+ secreted into the lumen, one Na+ and one HCO3- are reabsorbed into the plasma. H+ is recycled so there is little net secretion of H+ at this stage. A further 10 - 15% of HCO3- is similarly reabsorbed in the thick ascending limb of the loop of Henle. In the early distal tubule, H+ secretion is predominantly by Na+/H+ exchange but more distally, the Na+ gradient is insufficient so secretion is via H+ ATPase and H+/K+ ATPase in intercalated cells, which contain plentiful carbonic acid. As secreted H+ is derived from CO2, new HCO3- is formed and returns to the blood. H+ secretion is proportional to intracellular [H+] which itself is related to extracellular pH. A fall in blood pH will therefore stimulate renal H+ secretion. In the proximal tubule secretion of H+ serves to reclaim bicarbonate from glomerular filtrate so it is not lost, but in the distal nephron, secretion leads to net acid excretion and generation of new bicarbonate.Renal Regulation of Acid-Base Balance
Physiology / Renal / Acid-Base Balance
Last Updated: 26th July 2024
The pH of arterial blood is normally 7.35 - 7.45 ([H+] = 35 - 45 nmol/L).
Metabolism produces ~ 60 mmol H+ per day, most of which is excreted through the lungs as CO2, formed by the reaction of H+ with HCO3-. The kidneys conserve and replace HCO3- lost in this way, and fine tune H+ excretion. Physiological buffers maintain a low free [H+] and prevent large swings in pH.
Buffers
Buffers are weak acids or bases that can donate or accept H+ ions respectively and therefore resist changes in pH. Buffering does not alter the body's overall H+ load, ultimately the body must get rid of H+ by renal excretion if the buffering capacity of the body is not to be exceeded and a dangerous pH reached.
At a given [H+], a defined amount of buffer exists as acid (HA) and a defined amount as base (A-). The ratio of buffer pairs at a given [H+] is defined by the dissociation constant (pK) for that buffer pair. For a given acid-base pair, altering the ratio of the acid to the base alters the pH.
pK = ([H+][A-])/[HA], or pH = pK + log([A-]/[HA] (the Henderson-Hasselbalch equation).
Thus an increase in [A-] or a decrease in [HA] will increase pH and a decrease in pH will decrease the ratio [A-]/[HA].
Bicarbonate and carbonic acid (formed by the combination of CO2 with water, potentiated by carbonic anhydrase) are the most important buffer pair in the body, although haemoglobin provides about 20% of buffering in the blood, and phosphate and proteins provide intracellular buffering. Buffers in urine, largely phosphate, allow the excretion of large quantities of H+.
The bicarbonate system is physiologically effective because CO2 and HCO3- are precisely controlled by the lungs and the kidneys respectively.
Proximal Tubule
Bicarbonate is freely filtered at the glomerulus. Less than 0.1% of filtered bicarbonate is normally excreted in the urine (if plasma [HCO3-] increases, maximum tubular transport is exceeded and some HCO3- is excreted in urine). About 80% of bicarbonate is reabsorbed in the proximal tubule.
HCO3- is not transported directly, tubular HCO3- associates with H+ secreted by epithelial Na+/H+ antiporters to form carbonic acid (H2CO3) which readily dissociates to form carbon dioxide and water in the presence of carbonic anhydrase. CO2 and water diffuse into the tubular cells, where they recombine to form carbonic acid which dissociates to H+ and HCO3-.
This HCO3- is transported into the interstitium largely by Na+/HCO3- symporters on the basolateral membrane (and H+ is secreted back into the lumen). For each H+ secreted into the lumen, one Na+ and one HCO3- are reabsorbed into the plasma. H+ is recycled so there is little net secretion of H+ at this stage. A further 10 - 15% of HCO3- is similarly reabsorbed in the thick ascending limb of the loop of Henle.

Bicarbonate Handling in the Proximal Tubule. (Image by M. Koeppen, via Wikimedia Commons)
Ammonia
The body can excrete acid by the urinary loss of H+ ions associated with a buffer (predominantly sodium phosphate) or by the excretion of H+ ions as ammonium ions.
Ammonia is produced in tubular cells by the metabolism of glutamine, which leads to the generation of HCO3- and glucose. NH3 diffuses into the tubular fluid, or as NH4+ is transported by the Na+/H+ antiporter. In the tubular fluid, NH3 gains H+ to form NH4+ which cannot diffuse through membranes. About 50% of NH4+ secreted by the proximal tubule is reabsorbed in the thick ascending limb, where it substitutes for K+ in the Na+/K+/2Cl- symporter and passes into the medullary interstitium. Here NH4+ dissociates into NH3 and H+ and NH3 re-enters the collecting duct by diffusion. The secretion of H+ in the collecting duct leads to conversion back to NH4+ which is trapped in the lumen and excreted.
Distal Tubule
The secretion of H+ in the distal tubule promotes the reabsorption of any remaining HCO3-. The combination of H+ with NH3 and phosphate prevents H+ recycling and allows net acid excretion.
In the early distal tubule, H+ secretion is predominantly by Na+/H+ exchange but more distally, the Na+ gradient is insufficient so secretion is via H+ ATPase and H+/K+ ATPase in intercalated cells, which contain plentiful carbonic acid. As secreted H+ is derived from CO2, new HCO3- is formed and returns to the blood.
H+ secretion is proportional to intracellular [H+] which itself is related to extracellular pH. A fall in blood pH will therefore stimulate renal H+ secretion. In the proximal tubule secretion of H+ serves to reclaim bicarbonate from glomerular filtrate so it is not lost, but in the distal nephron, secretion leads to net acid excretion and generation of new bicarbonate.
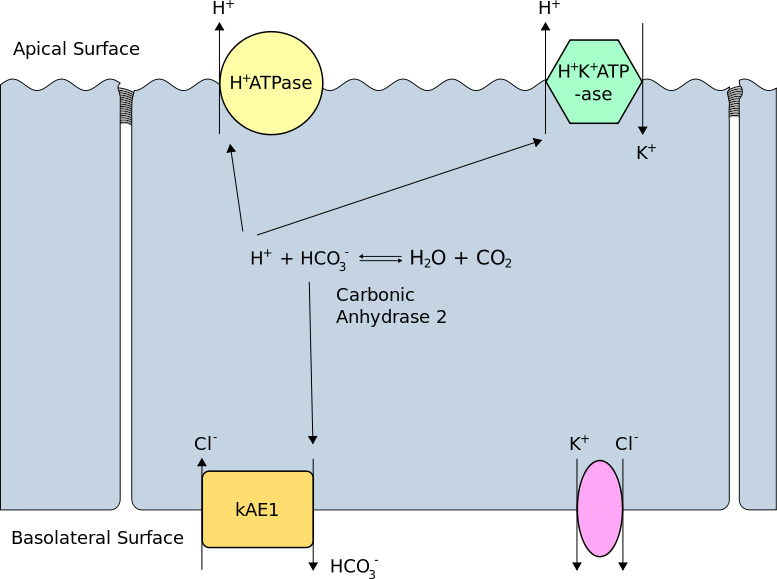
Secretion of Hydrogen Ions in the Distal Tubule. (Image by Rswarbrick [CC BY 3.0 , via Wikimedia Commons)
Report A Problem
Is there something wrong with this question? Let us know and we’ll fix it as soon as possible.
Loading Form...
- Biochemistry
- Blood Gases
- Haematology
| Biochemistry | Normal Value |
|---|---|
| Sodium | 135 – 145 mmol/l |
| Potassium | 3.0 – 4.5 mmol/l |
| Urea | 2.5 – 7.5 mmol/l |
| Glucose | 3.5 – 5.0 mmol/l |
| Creatinine | 35 – 135 μmol/l |
| Alanine Aminotransferase (ALT) | 5 – 35 U/l |
| Gamma-glutamyl Transferase (GGT) | < 65 U/l |
| Alkaline Phosphatase (ALP) | 30 – 135 U/l |
| Aspartate Aminotransferase (AST) | < 40 U/l |
| Total Protein | 60 – 80 g/l |
| Albumin | 35 – 50 g/l |
| Globulin | 2.4 – 3.5 g/dl |
| Amylase | < 70 U/l |
| Total Bilirubin | 3 – 17 μmol/l |
| Calcium | 2.1 – 2.5 mmol/l |
| Chloride | 95 – 105 mmol/l |
| Phosphate | 0.8 – 1.4 mmol/l |
| Haematology | Normal Value |
|---|---|
| Haemoglobin | 11.5 – 16.6 g/dl |
| White Blood Cells | 4.0 – 11.0 x 109/l |
| Platelets | 150 – 450 x 109/l |
| MCV | 80 – 96 fl |
| MCHC | 32 – 36 g/dl |
| Neutrophils | 2.0 – 7.5 x 109/l |
| Lymphocytes | 1.5 – 4.0 x 109/l |
| Monocytes | 0.3 – 1.0 x 109/l |
| Eosinophils | 0.1 – 0.5 x 109/l |
| Basophils | < 0.2 x 109/l |
| Reticulocytes | < 2% |
| Haematocrit | 0.35 – 0.49 |
| Red Cell Distribution Width | 11 – 15% |
| Blood Gases | Normal Value |
|---|---|
| pH | 7.35 – 7.45 |
| pO2 | 11 – 14 kPa |
| pCO2 | 4.5 – 6.0 kPa |
| Base Excess | -2 – +2 mmol/l |
| Bicarbonate | 24 – 30 mmol/l |
| Lactate | < 2 mmol/l |

